Soy Food Consumption Is Associated with Lower Risk of Coronary Heart Disease in Chinese Women (note 1). This article was extracted from “The Journal of Nutrition, jn.nutrition.org”
Xianglan Zhang, Xiao Ou Shu, (note 2) Yu-Tang Gao,* Gong Yang, Qi Li,* Honglan Li,* Fan Jin* and Wei Zheng
Department of Medicine, Center for Health Services Research, Vanderbilt University, Nashville, TN 37232 and *Department of Epidemiology, Shanghai Cancer Institute, Shanghai 200032, China
It has been postulated that high consumption of soy foods may be one of the possible explanations for the low risk of coronary heart disease (CHD)3 in some Asian populations (1,2). Several potential mechanisms through which soy and soy constituents may protect against CHD have been proposed (2–9), including the well-documented beneficial effects of soy on serum lipid profiles (10–14) and other lipid-independent effects, such as lowering blood pressure (14–16), increasing LDL oxidation resistance (17–19), improving vascular reactivity (20–23), inhibiting thrombus formation and suppressing smooth muscle cell proliferation and migration (24, 25). A meta-analysis of 38 controlled clinical trials reported that intake of soy protein was associated with a significant reduction in serum concentrations of total cholesterol, LDL cholesterol and triacylglycerols, and a nonsignificant increase in HDL cholesterol (10).
Despite the strong evidence for potential beneficial effects of soy foods on cardiovascular disease (CVD) risk factors or surrogate end points (10–28), no prospective study has directly assessed the association between usual dietary soy intake and risk of CHD. The Shanghai Women’s Health Study (SWHS), conducted in a population with a high soy food intake, provides a unique opportunity with which to evaluate this important hypothesis.
SUBJECTS AND METHODS
Study population. The SWHS is a population-based prospective cohort study conducted among Chinese women in urban Shanghai. Eligible participants were female permanent residents of Shanghai, aged 40–70 y, who were living in seven urban communities of Shanghai. The study was approved by relevant institutional review boards for human research in Shanghai and the United States. Of 81,271 eligible women identified from the Shanghai residential registry, 2047 (3.0%) refused to participate in the study, 2073 (2.6%) were not available during the baseline recruitment period and 1469 (1.8%) were not enrolled for other miscellaneous reasons related to health and communication problems. The remaining 75,322 women completed the baseline survey during 1997–2000 and were recruited to the cohort study, yielding a participation rate of 92.7%. After further exclusion of those who were later found not to be in the age range at the time of interview, the final SWHS cohort consisted of 75,044 women.
The baseline in-person interviews were performed at participants’ homes by trained interviewers using a structured questionnaire designed to collect information on demographic characteristics, diet and lifestyle habits, and medical history. Anthropometric measurements, including current weight, height, and circumferences of waist and hip, were taken using a standard protocol. All interviews were tape-recorded and selectively checked by quality control staff members to monitor the quality of the interview data. All study participants were followed through biennial in-person interviews.
Note 1. Supported by National Institutes of Health grant R01CA70867.
Note 2. To whom correspondence and reprint requests should be addressed. E-mail: Xiao-Ou.Shu@Vanderbilt.edu.
Note 3. Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; FFQ, food-frequency questionnaire; MI, myocardial infarction; RR, relative risk; SWHS, Shanghai Women’s Health Study.
For the present study, we excluded women who reported a history of CHD, stroke or cancer at the time of the baseline interview (n = 7409). Those with previously diagnosed diabetes were also excluded because diagnosis of this intermediate condition of CHD would likely lead to a dietary change and thus may confound the diet-disease relationship (n = 2381). We also excluded women with an extreme total energy intake (<2092 or >14,644 kJ/d) (n = 105) and those who were lost to follow-up immediately after initial entry (n = 239). After these exclusions, a total of 64,915 women remained for the present analysis.
Dietary assessment. Usual dietary intake was assessed through an in-person interview using a comprehensive quantitative food-frequency questionnaire (FFQ). The FFQ included tofu, soymilk, fried bean curd, bean curd cake and other soy products, covering virtually all soy foods consumed in urban Shanghai. During the face-to-face interview, each participant was first asked how often, on average, during the previous year she had consumed a specific food or food group (the possible responses were daily, weekly, monthly, yearly or never), followed by a question on the amount consumed in grams per unit of time. The intake of nutrients was then calculated by multiplying the amount of food consumed by the nutrient contents per gram of food obtained from the Chinese Food Composition Table (29). Total soy food intake was measured by summing up the soy protein intake for all soy food items.
The FFQ was evaluated for its reproducibility and validity in assessing usual dietary intake in a random sample of 200 SWHS participants (30). The median intakes of protein derived from the FFQ and the 24-h dietary survey were very similar (60.8 and 61.3 g/d, respectively). At the individual level, the estimate of protein intake derived from the FFQ was closely correlated with that derived from the survey with 24 d of 24-h dietary recalls (Pearson correlation coefficient, r = 0.60, P < 0.01).
End point ascertainment. The primary end point for this study was incident CHD, including both nonfatal myocardial infarction (MI) and CHD death, that occurred after the baseline survey. Cases of nonfatal MI were identified through in-person follow-up interviews that were conducted typically 2 y after the baseline survey. Medical records were sought for all self-reported cases of MI and reviewed by physicians who were unaware of the participant’s exposure status. The diagnosis of MI was confirmed using the WHO criteria (i.e., symptoms plus either diagnostic electrocardiographic changes or elevation of cardiac enzyme levels) (31). Cases of MI were defined as possible when information from medical records was insufficient or unavailable and confirmatory information was obtained through an in-person interview only. There were 10 (23%) possible MI cases.
Deaths were ascertained through reports by the next of kin and linkage with the registry of vital statistics kept at the Shanghai Center for Disease Control and Prevention. Virtually all cohort members (99.6%) were successfully followed for their vital status by means of the biennial home visits in conjunction with administrative data. The underlying causes of death were established by reviewing medical records when possible, interviewing the next of kin to find out the circumstances of death and the major medical histories of the decedents, and by referring to death certificates. Of the 19 CHD deaths identified during the follow-up period, seven were confirmed to be fatal MI cases by means of medical record reviews, 12 were presumed to be attributed to CHD for which CHD was the most plausible cause of death and no other known causes of death could be suggested based on interviews and information from death certificates in which the underlying cause of death was recorded as an International Classification of Diseases, 9th Revision (ICD-9) code of 410 to 414. All possible MI cases and presumed CHD deaths were included in this analysis after ensuring that the results would not vary substantially by inclusion or exclusion of these cases.
Statistical analysis. Person-years of follow-up for each participant were calculated from the date of the baseline interview to the date of the end point, death, or the follow-up survey, whichever came first. The participants were divided into four categories, according to the quartile distribution of total soy protein intake at baseline among the entire cohort. The lowest quartile was treated as the reference group. Incidence rates were calculated by dividing the number of events by the person-years of follow-up in each category. Relative risks (RR), the rate ratios of each specific quartile vs. the lowest quartile, and their 95% CI were computed using the Cox proportional hazards model to measure the association between consumption of soy foods and the risk of CHD. Selection of potential confounders was based primarily on a priori consideration of their associations with both soy intake and CHD risk as well as the change-in-estimate criterion (comparing unadjusted and adjusted estimates of relative risks). Variables adjusted for in the multivariate analyses included age, cigarette smoking, alcohol consumption, BMI (kg/m2), waist to hip ratio, regular exercise, menopausal status, history of hypertension, attended education level, family income, season of recruitment, as well as intake of total energy, fat, fiber, fruit and vegetables. Additional adjustment for use of postmenopausal hormones, vitamin E supplement, multivitamins and other dietary intakes (e.g., meat, poultry, fish, egg, rice and tea) did not materially alter the results; these variables were therefore not included in the final model. Tests for linear trend across the quartiles were performed by using the median intake value for each category and modeling them as continuous variables. We also conducted analyses stratified by known CVD risk factors and some dietary factors to further examine the independent effects of soy and to explore the possible presence of effect modification by these factors.
The “proportionality assumption” that underlies the Cox model was checked by graphical methods and was found not to have been violated. All P-values are two-sided. Statistical analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC).
RESULTS
A total of 64,915 women were included in the current analysis. The mean age of the cohort members was 51 y at recruitment. The mean length of follow-up was 2.5 y. The median dietary intake of total soy protein was 7.36 g/d. The major contributors to total soy protein intake in the study population were soy milk, tofu and processed soy products other than tofu, accounting for 81% of total soy protein intake.
We examined the baseline characteristics of study participants according to quartiles of total soy protein intake (Table 1). Compared with women with lower soy protein consumption, women with higher consumption were older, more likely to be postmenopausal, and have a history of hypertension, a higher BMI and waist-to-hip ratio, characteristics that relate to aging. Women who ate more soy protein were also more likely to exercise and drink alcohol, and less likely to smoke cigarettes. Soy protein intake was positively associated with intake of total energy, fat, fiber and vegetables. No associations with education and family income were observed for soy protein intake.
During the 162,277 person-years of follow-up, 62 incident cases of CHD (43 nonfatal MI cases and 19 deaths from CHD) were documented. Relative risks and 95% CI for CHD according to quartiles of total soy protein intake were calculated (Table 2). The age- and energy-adjusted risk of total CHD decreased with increasing total soy protein intake (P for trend = 0.03). The inverse association was strengthened after further adjusting for risk factors for CVD, socioeconomic and dietary factors. Compared with women in the lowest quartile of total soy protein intake, the RR of CHD derived from the fully adjusted model was 0.25 (95% CI, 0.10–0.63; P for trend = 0.003) for women in the highest quartile of intake. A stronger inverse association was observed for nonfatal MI with a multivariate RR for the highest quartile being 0.14 (95% CI, 0.04–0.48; P for trend = 0.001). No association was found for fatal CHD, likely due to a small number of outcomes. Analyses restricted to confirmed cases of nonfatal MI and fatal CHD had similar results (Table 2).

TABLE 1
Baseline characteristics of the study population according to quartiles of total soy protein intake, the Shanghai Women’s Health Study (1997–2002)
1 Values are means ± SD for continuous variables and percentage of subjects for categorical variables.
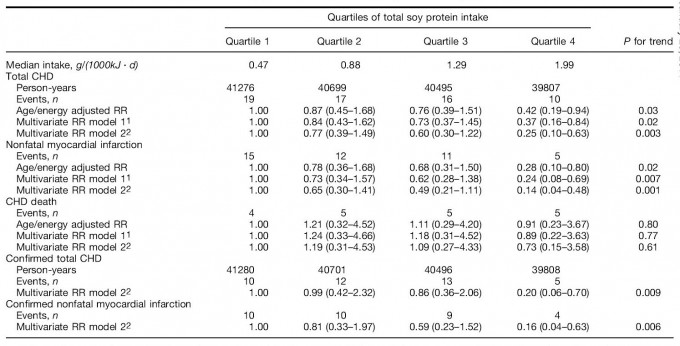
TABLE 2
Relative risks (RR) and 95% CI of coronary heart disease (CHD) by quartiles of total soy protein intake, the Shanghai Women’s Health Study (1997–2002)
1 Model 1 adjusted for age (continuous), cigarette smoking (yes or no), BMI (quartile), waist-to-hip ratio (quartile), history of hypertension (yes or no), menopausal status (pre- or postmenopausal), regular exercise (yes or no), level of education (four categories), family income (four categories), alcohol consumption (yes or no), season of recruitment (four categories) and total energy intake (continuous).
2 Model 2 additionally adjusted for intakes of fat, fiber, fruit and vegetables (all treated as continuous variables).
In further analyses stratified by hypertension, BMI, waist to hip ratio, exercise, menopausal status and intakes of fat, fiber, fruit and vegetables, the inverse association between soy protein intake and CHD risk was observed consistently across all strata (Table 3). The magnitude of the stratum-specific relative risks comparing the extreme tertiles did not differ substantially for all of these factors, indicating that there was no significant effect modification.
DISCUSSION
In this population-based, prospective cohort study, we found a significant inverse association between soy food intake and risk of CHD. Women in the highest quartile of soy protein intake had a 75% lower risk of total CHD and 86% lower risk of nonfatal MI than those in the lowest quartile. This association was independent of established CVD risk factors and other dietary factors.
The observed inverse association of soy food intake with CHD risk is biologically plausible. Soy food intake improves serum lipid and lipoprotein profiles (10). Such well-established beneficial effects have led to an approval of health claims on food labels by the U.S. FDA (32) and a recent dietary recommendation from the Nutrition Committee of the American Heart Association (33). Other potential mechanisms have also drawn much attention recently. A randomized controlled trial reported that a 3-mo soymilk diet significantly lowered blood pressure among essential hypertensive patients (16). It has also been shown that 2 wk of soy consumption by six volunteers significantly prolonged LDL oxidation lag time (17), suggesting an increased resistance of LDL particles to oxidation, a key event in early atherogenesis. One study in a Dutch population found that higher dietary intake of isoflavones, for which soy is the richest food source, was associated with lower aortic stiffness (23), an indicator of cardiovascular health. Two studies of postmenopausal women in the U.S. examined the association of usual dietary isoflavone intake with several risk factors for CVD, including serum lipids and lipoproteins, blood pressure, BMI, body fat distribution and glucose metabolism, and suggested a cardioprotective potential of dietary soy intake (26,27). Experimental studies with cynomolgus monkeys have also provided direct evidence that soy isoflavones inhibit atherosclerosis (34,35).
Data on the direct relationship of soy to clinical outcomes of CVD are lacking. One recently published study (36) in Japan found a significant inverse association between intake of soy and total mortality. The association with specific causes of death including CVD, however, was not significant, perhaps due to a small sample size. To our knowledge, our study is the first one to prospectively assess the relationship between soy food intake and the incidence of CHD, providing direct evidence for the potential role of soy in the prevention of CHD.
Other strengths of our study include a population-based study design with a high participation rate and a virtually complete cohort follow-up, a large sample size, a high and diverse intake level of soy foods and the use of a face-to-face interview. The comprehensive information collected at baseline also allowed us to adjust for important nondietary and dietary factors that may confound the soy foods and CHD relationship.
One of the major concerns for this observational study is the potential error in dietary assessment. The FFQ used in this study, however, was validated for its ability to estimate soy food intake (30). Furthermore, because of the prospective design of this study, the potential error in dietary assessment is likely to be random, which tends to bias the results toward the null.
Another potential concern is that there might be some misclassification in outcome ascertainment. For some self-reported cases, the diagnosis could not be confirmed because their medical records were incomplete or unavailable. However, we conducted the analyses separately by both including and excluding these possible cases and found that the results did not vary materially.
Although we could not completely rule out the possibility of the presence of residual confounding, we used several approaches such as restriction, multivariate modeling and stratification to control for the effects of potential confounders in our study. A consistent, strong and graded inverse association persisted in all analyses, suggesting that confounding was unlikely to be an attributor.
Finally, the short length of follow-up raised the concern that some subjects may have modified their diets because of early symptoms of undiagnosed CHD. We therefore conducted a sensitivity analysis by excluding the cases that occurred during the first 6 mo or the first 12 mo of follow-up, and found little change in the risk estimates.

TABLE 3
Multivariate relative risks (RR) of coronary heart disease by tertiles of total soy protein intake, stratified by selected factors, the Shanghai Women’s Health Study (1997–2002)
1 Adjusted for age (continuous), cigarette smoking (yes or no), BMI (quartile), waist-to-hip ratio (quartile), history of hypertension (yes or no), menopausal status (pre- or postmenopausal), regular exercise (yes or no), level of education (four categories), family income (four categories), alcohol consumption (yes or no), season of recruitment (four categories), total energy intake, and intakes of fat, fiber, fruit and vegetables (all treated as continuous variables). When a variable was used for stratification, it was not included in the model.
2 Median value was used as the cut-off point.
In summary, we found in this large prospective cohort study that soy food consumption was significantly and inversely associated with the risk of CHD among Chinese women. Our study provides the strongest argument to date for the recommendation made by the American Heart Association to increase soy food intake to promote heart health.
Best,
Tan Kok Hui
Nutrition Made Simple, Life Made Rich
LITERATURE CITED
- Adlercreutz, H. & Mazur, W. (1997) Phyto-oestrogens and Western diseases. Ann. Med. 29: 95–120.
- Tikkanen, M. J. & Adlercreutz, H. (2000) Dietary soy-derived isoflavone phytoestrogens. Could they have a role in coronary heart disease prevention? Biochem. Pharmacol. 60: 1–5.
- Lichtenstein, A. H. (1998) Soy protein, isoflavones and cardiovascular disease risk. J. Nutr. 128: 1589–1592.
- Anthony, M. S., Clarkson, T. B. & Williams, J. K. (1998) Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am. J. Clin. Nutr. 68 (suppl. 6): 1390S–1393S.
- Lissin, L. W. & Cooke, J. P. (2000) Phytoestrogens and cardiovascular health. J. Am. Coll. Cardiol. 35: 1403–1410.
- Anthony, M. S. (2000) Soy and cardiovascular disease: cholesterol lowering and beyond. J. Nutr. 130: 662S–663S.
- Vitolins, M. Z., Anthony, M. & Burke, G. L. (2001) Soy protein isofla-vones, lipids and arterial disease. Curr. Opin. Lipidol. 12: 433–437.
- Sirtori, C. R. & Lovati, M. R. (2001) Soy proteins and cardiovascular disease. Curr. Atheroscler. Rep. 3: 47–53.
- Clarkson, T. B. (2002) Soy, soy phytoestrogens and cardiovascular disease. J. Nutr. 132: 566S–569S.
- Anderson, J. W., Johnstone, B. M. & Cook-Newell, M. E. (1995) Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 333: 276–282.
- Wong, W. W., Smith, E. O., Stuff, J. E., Hachey, D. L., Heird, W. C. & Pownell, H. J. (1998) Cholesterol-lowering effect of soy protein in normocho-lesterolemic and hypercholesterolemic men. Am. J. Clin. Nutr. 68 (suppl. 6): 1385S–1389S.
- Merz-Demlow, B. E., Duncan, A. M., Wangen, K. E., Xu, X., Carr, T. P., Phipps, W. R. & Kurzer, M. S. (2000) Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am. J. Clin. Nutr. 71: 1462–1469.
- Wangen, K. E., Duncan, A. M., Xu, X. & Kurzer, M. S. (2001) Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am. J. Clin. Nutr. 73: 225–231.
- Washburn, S., Burke, G. L., Morgan, T. & Anthony, M. (1999) Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause. 6: 7–13.
- Teede, H. J., Dalais, F. S., Kotsopoulos, D., Liang, Y. L., Davis, S. & McGrath, B. P. (2001) Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J. Clin. Endocrinol. Metab. 86: 3053–3060.
- Rivas, M., Garay, R. P., Escanero, J. F., Cia, P., Jr., Cia, P. & Alda, J. O. (2002) Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J. Nutr. 132: 1900–1902.
- Tikkanen, M. J., Wahala, K., Ojala, S., Vihma, V. & Adlercreutz, H. (1998) Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc. Natl. Acad. Sci. U.S.A. 95: 3106–3110.
- Wiseman, H., O’Reilly, J. D., Adlercreutz, H., Mallet, A. I., Bowey, E. A., Rowland, I. R. & Sanders, T. A. (2000) Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 72: 395–400.
- Jenkins, D. J., Kendall, C. W., Vidgen, E., Vuksan, V., Jackson, C. J., Augustin, L. S., Lee, B., Garsetti, M. & Agarwal, S., et al. (2000) Effect of soy-based breakfast cereal on blood lipids and oxidized low-density lipoprotein. Metabolism 49: 1496–1500.
- Nestel, P. J., Yamashita, T., Sasahara, T., Pomeroy, S., Dart, A., Komesaroff, P., Owen, A. & Abbey, M. (1997) Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler. Thromb. Vasc. Biol. 17: 3392–3398.
- Walker, H. A., Dean, T. S., Sanders, T. A., Jackson, G., Ritter, J. M. & Chowienczyk, P. J. (2001) The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 178-estradiol. Circulation 103: 258–262.
- Yildirir, A., Tokgozoglu, S. L., Oduncu, T., Oto, A., Haznedaroglu, I., Akinci, D., Koksal, G., Sade, E., Kirazli, S. & Kes, S. (2001) Soy protein diet significantly improves endothelial function and lipid parameters. Clin. Cardiol. 24: 711–716.
- van der Schouw, Y. T., Pijpe, A., Lebrun, C. E., Bots, M. L., Peeters, P. H., van Staveren, W. A., Lamberts, S. W. & Grobbee, D. E. (2002) Higher usual dietary intake of phytoestrogens is associated with lower aortic stiffness in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 22: 1316–1322.
- Wilcox, J. N. & Blumenthal, B. F. (1995) Thrombotic mechanisms in atherosclerosis: potential impact of soy proteins. J. Nutr. 125 (suppl. 3): 631S– 638S.
- Raines, E. W. & Ross, R. (1995) Biology of atherosclerotic plaque formation: possible role of growth factors in lesion development and the potential impact of soy. J. Nutr. 125 (suppl. 3): 624S–630S.
- Goodman-Gruen, D. & Kritz-Silverstein, D. (2001) Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J. Nutr. 131: 1202–1206.
- de Kleijn, M. J., van der Schouw, Y. T., Wilson, P. W., Grobbee, D. E. & Jacques, P. F. (2002) Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U. S. women: the Framingham study. J. Nutr. 132: 276–282.
- Scheiber, M. D., Liu, J. H., Subbiah, M. T., Rebar, R. W. & Setchell, K. D. (2001) Dietary inclusion of whole soy foods results in significant reductions in clinical risk factors for osteoporosis and cardiovascular disease in normal postmenopausal women. Menopause 8: 384–392.
- Wang, G. Y. & Shen, Z. P., eds. (1991) Chinese Food Composition Tables. People’s Health Publishing House, Beijing, China.
- Shu, X. O., Yang, G., Jin, F., Liu, D. K., Kushi, L., Wei, W. Q., Gao, Y. T. & Zheng, W. (2003) Validity and reproducibility of the food frequency ques-tionnaire used in the Shanghai Women’s Health Study. Eur. J. Clin. Nutr. (in press).
- Rose, G. A. & Blackburn, H. (1982) Cardiovascular Survey Methods. WHO monograph series, no. 58. World Health Organization, Geneva, Switzerland.
- Food and Drug Administration. (1999) Food labeling: health claims; soy protein and coronary heart disease. Fed. Reg. 64: 57699–57733.
- Erdman, J. W., Jr. (2000) AHA Science Advisory: Soy protein and cardiovascular disease: a statement for healthcare professionals from the Nutrition Committee of the AHA. Circulation 102: 2555–2559.
- Anthony, M. S., Clarkson, T. B., Bullock, B. C. & Wagner, J. D. (1997) Soy protein versus soy phytoestrogens in the prevention of diet-induced coronary artery atherosclerosis of male cynomolgus monkeys. Arterioscler. Thromb. Vasc. Biol. 17: 2524–2531.
- Clarkson, T. B., Anthony, M. S. & Morgan, T. M. (2001) Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J. Clin. Endocrinol. Metab. 86: 41–47.
- Nagata, C., Takatsuka, N. & Shimizu, H. (2002) Soy and fish oil intake and mortality in a Japanese community. Am. J. Epidemiol. 156: 824–831.






